Carmine Therapeutics is developing allogeneic, redosable, non-viral gene therapies to treat diseases for which a high unmet medical need exists. The company’s technology harnesses the natural properties of human red blood cells (RBCs) to produce extracellular vesicles (EVs), allowing the delivery of large payloads of genetic material, resulting in high-amplitude, durable transgene expression to replace missing or mutated genes found within the patient. The initial focus is on ocular diseases, including Stargardt disease with plans to expand to cystic fibrosis and neurological diseases in the future.
Gene therapy is a promising field of medicine that has the potential to cure diseases by altering or replacing faulty genes that cause illness. It involves using genetic material to edit a faulty copy or deliver healthy copies of a gene to cells in the body. This has the potential to treat a wide range of diseases, including rare genetic disorders, cancer, and degenerative diseases. However, a primary challenge of gene therapy is transporting the genetic material into the cells. Common techniques are to use transfected cells or viruses, but host-defense mechanisms can result in poor engraftment or dwindling production from the transplanted cells.
Lipid nanoparticles (LNPs) are another way to deliver genetic material into cells, and LNPs do a fine job of protecting the genetic material from degradation by host defense. However, there are a few potential downsides to using LNPs for gene therapy. Firstly, LNPs may not be able to effectively deliver gene therapy to all types of cells, particularly non-dividing cells or cells that are deep within the body. Additionally, LNPs have limited payload capacity that restricts their usefulness for certain types of diseases. Finally, there may be toxicity concerns associated with using LNPs for gene therapy, as they are typically composed of synthetic materials that may not be well-tolerated by the body.
Carmine Therapeutics is pioneering the use of extracellular vesicles derived from RBCs (RBCEVs) for gene therapy delivery. These allogeneic RBCEVs are derived from O-negative “universal donor” RBCs, and thus can be given to any patient, and can also be re-dosed. The REGENT® platform takes advantage of several unique characteristics of RBCs, including a well-understood safety profile dating back over 100 years, lack of inflammatory response, large payload capacity, durable transgene expression, scalable manufacturing, and favorable commercial logistics.
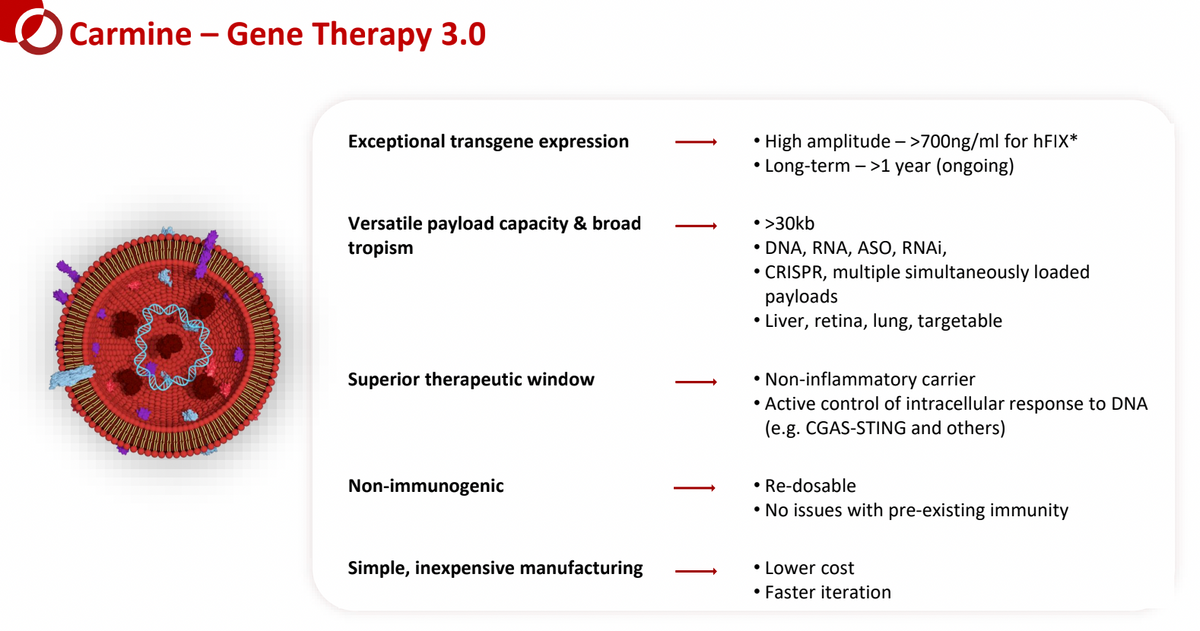
Carmine’s preclinical data show:
- High amplitude DNA transgene expression: Mice intravenously treated with RBCEVs (hFIX DNA) show >700 nanograms per mL of protein expression. That’s roughly 6X therapeutic threshold.
- Durable, long-term transgene expression: Mice show durable expression out over one year. RBCEVs also have the potential for redosing, which is generally considered not possible with AAV gene therapy due to the formation of neutralizing antibodies.
- Exceptionally large and versatile payloads: Perhaps the most attractive characteristic of the REGENT platform is the ability to include payloads in excess of >30 kb, 5X that of AAV, as well as the ability to include multiple genetic payloads inside a single EV. Furthermore, EVs can carry a versatile payload composition of DNA, RNA, ASO, and RNAi. This kind of platform flexibility allows for targeting some opportunities with gene therapy constructs not previously achievable with AAV or LNP delivery.
- Broad tropism and flexible administration: RBCEVs can reach multiple different organ types, including the liver, retina, lung, or even CNS. Furthermore, RBCEVs can be designed to target organs of choice. Carmine has preclinical proof-of-concept data showing administration via intravenous, intrathecal, subretinal, and inhaled (nebulized) delivery. This allows for broad-scale clinical development targeting multiple genetic diseases.
- Safety and tolerability: Preclinical data show no liver toxicity (ALT/AST elevation), with minimal and transient cytokine release. And importantly, Carmine believes that its RBCEVs gene therapies can be dosed without the need for preconditioning regimens (steroids and other anti-inflammatory agents) required prior to patients receiving other types of gene therapy. This opens the door to treating patients with weakened or compromised immune systems or those in later-stage disease who might not be able to tolerate alternative approaches.
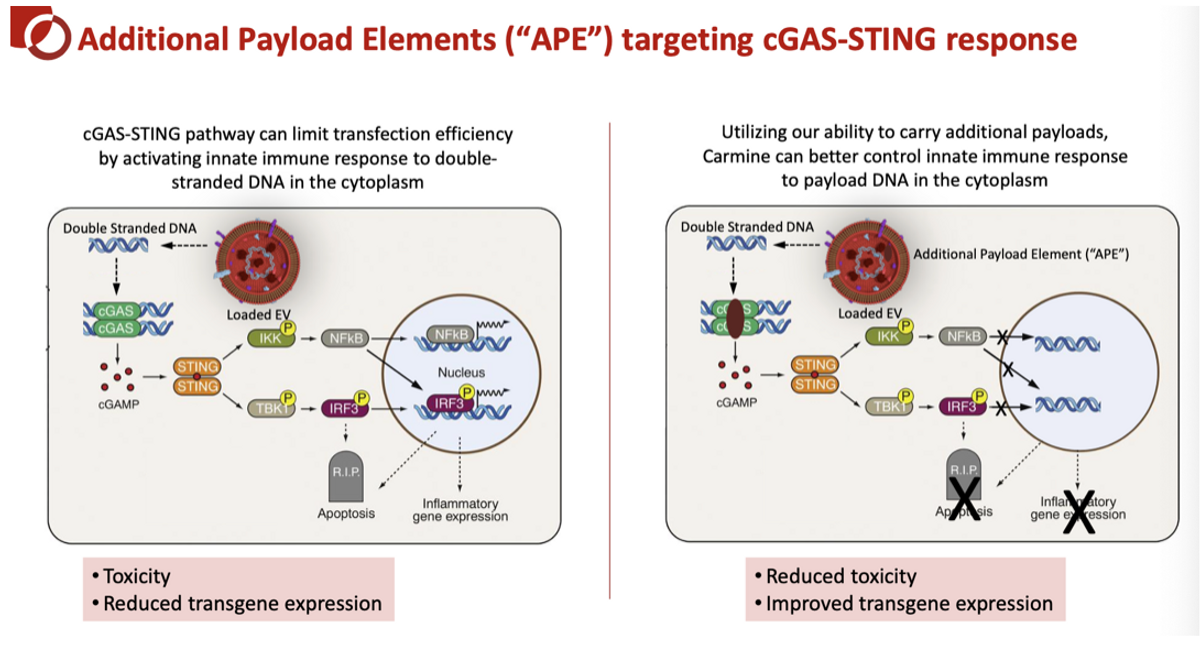
- Decreasing DNA payload related toxicity: Carmine has preclinical proof-of-concept data showing that the inclusion of “additional payload elements” (APE) can target and block the cGAS-STING response, further improving tolerability while allowing for increased dose optimization that leads to greater and more durable transgene expression.
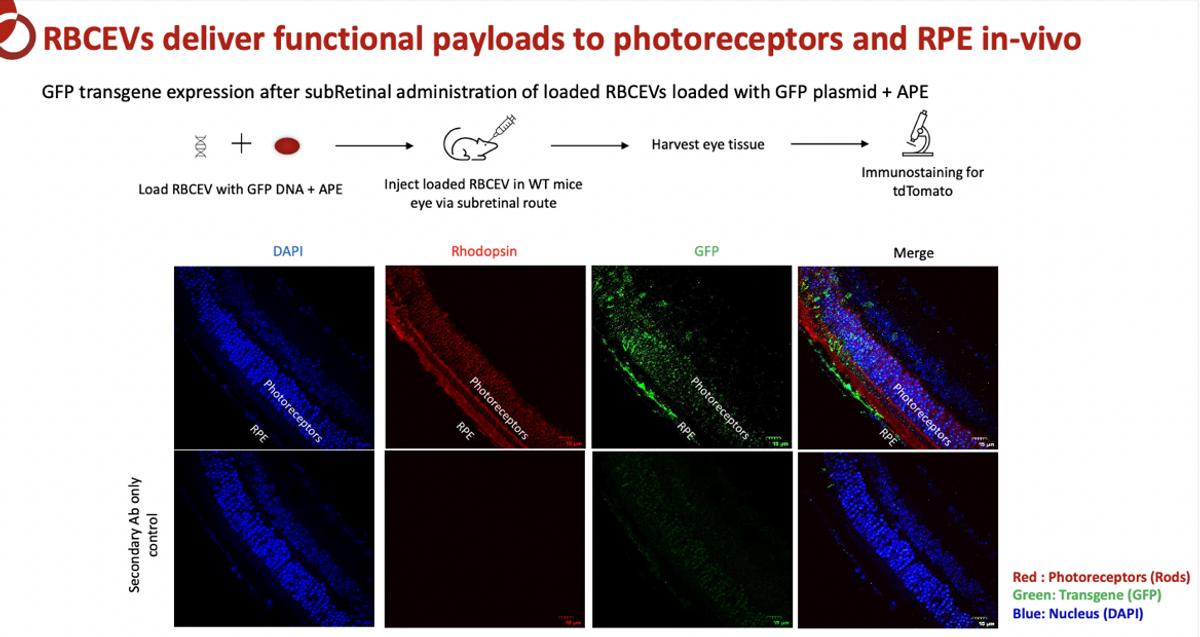
Carmine’s initial focus is on Stargardt disease (STGD1), the most common inherited retinal dystrophy in children and adults. STGD1 is the result of a dysfunctional retina-specific gene, ABCA4, which causes massive accumulation of toxic vitamin A byproducts in the retina leading to retinal cell death and progressive loss of central vision. The ABCA4 transgene of 6.8 kb exceeds the capacity of AAV vectors. Carmine is working on a subretinal delivery of RBCEVs that provide a working copy of the ABCA4 gene. Preclinical data show mice transfected with RBCEV candidates, along with Carmine’s proprietary APE payload, show GFP transgene production in the targeted retinal photoreceptors. Immunostaining for GFP transgene expression shows evidence of accumulation in the photoreceptors (rods), rhodopsin, and DAPI cells, suggesting Carmine’s drug candidate could have a highly relevant therapeutic effect.
Carmine also demonstrated preclinical proof-of-concept with a nebulizer formulation of RBCEVs carrying the tdTomato mRNA or DNA into the lungs of mice, suggesting target transgene expression in the respiratory epithelium, which could have profound therapeutic effects for the treatment of cystic fibrosis. Like Stargardt disease, the target transgene of the full length cystic fibrosis transmembrane conductance regulator (CFTR) protein exceeds the capacity of AAV vector gene therapy. And it is generally thought that inhaling LNPs would put further stress on the already weakened and deteriorated lung. Additionally, durability of expression has been a hurdle for gene therapy due to the high turnover rate of these respiratory epithelial cells. However, Carmine’s data suggests penetration into the deeper lung epithelial cells, where basal progenitor cells remain , suggesting potential of a more durable response. Furthermore, the lack of immunogenicity and inflammatory response to RBCEVs allows for potential repeat or chronic dosing - something we have yet to see with gene therapy candidates. This could potentially be a unique opportunity for Carmine in developing an effective, and redosable, gene therapy for cystic fibrosis.
The final favorable attribute of Carmine’s REGENT platform is the ease and efficiency of the manufacturing process. Carmine starts with whole blood purchased from suppliers and then it’s only a few day process to isolate and purify out the RBCEVs. Importantly, the empty EVs are shelf stable at -80°C through multiple cycles. After the EVs are purified, it’s only another few day process to load the payloads, which are also stable at -80°C through multiple cycles. While CMC logistics have plagued some cell and gene therapy competitors, Carmine’s straightforward, low-cost, scalable process, all of which is protected under Trade Secret and foundational IP, provides a solid competitive advantage over competitors.
Gene therapy holds significant promise for the treatment of rare diseases that are today either untreatable or carry significant treatment burden (i.e. enzyme replacement therapy). Carmine's scalable, redosable, non-viral approach has the potential to improve upon first-generation gene therapy candidates in terms of both safety and efficacy. And importantly, the team at Carmine is led by industry veterans with significant experience in gene therapy and therapeutic drug development, backed by a solid team of world-renowned scientific advisors and board of directors.